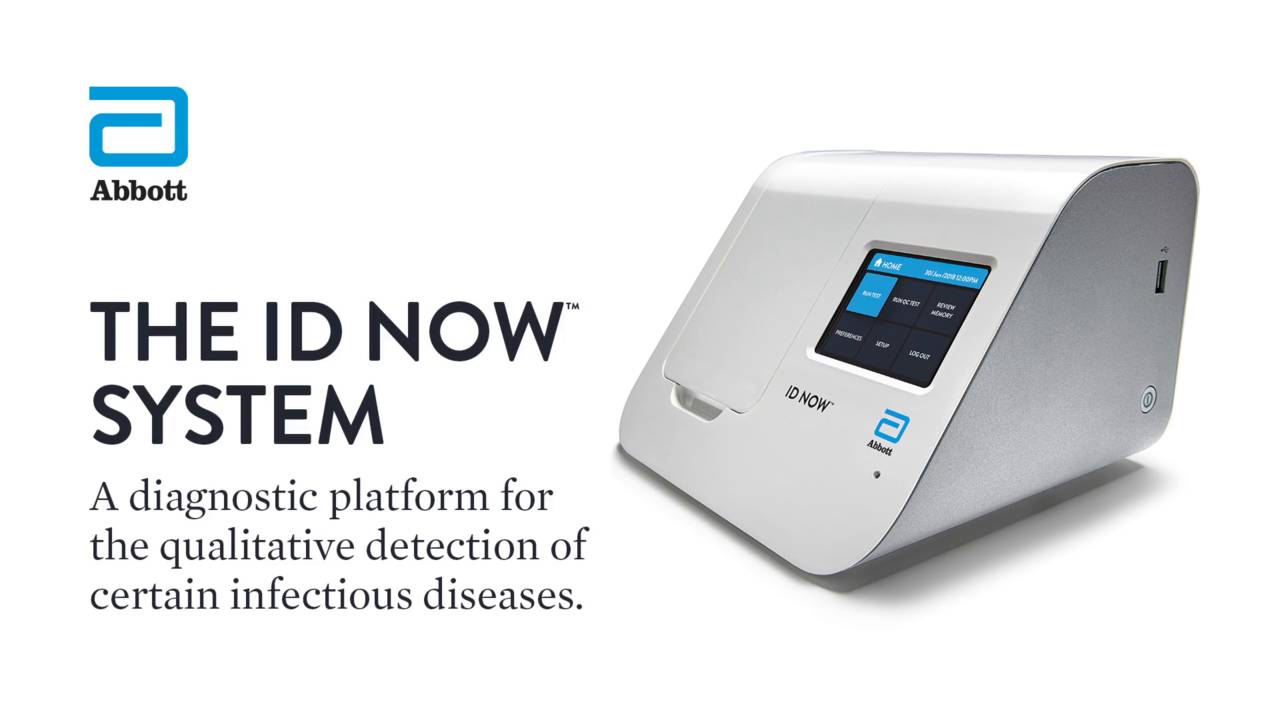
Description
The intuitively designed ID NOW™ instrument can have a positive impact in any healthcare setting.
- Easy to use with only minimal training requirements
- Large visual touchscreen displays results, eliminating transcription errors and the need for printing
- Eliminates interpretation and transcription errors
- Gives you the confidence to make clinical decisions sooner
- Facilitates effective patient management
- Aids targeted antiviral therapy and Antimicrobial Stewardship